
The Government Accountability Office (GAO) says the FDA should up its import seafood safety warning game. Mostly by monitoring and improving the FDA’s warning letter effectiveness.
The Food and Drug Administration ensures that imported seafood is safe to eat. If companies violate food safety regulations and pose a public health risk, FDA may send them warning letters.
For the letters that FDA sent from 2014-early 2019, FDA didn’t consistently follow key procedures or meet key goals. For example, FDA set a goal to conduct follow-up inspections within 6 months of issuing a warning letter to ensure violations were corrected. But FDA met this goal for just 14 of 125 warning letters sent for significant inspection violations.
They recommended that FDA monitor whether it’s following procedures and meeting goals for its warning letters.
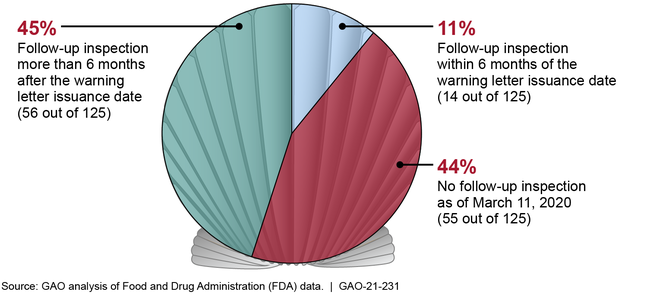
The Food and Drug Administration (FDA) issues warning letters for food safety violations that could pose a risk to public health. According to FDA, warning letters are its primary means of getting firms to voluntarily comply with food safety laws and regulations. GAO analyzed 167 imported seafood warning letters that FDA issued from January 1, 2014, through March 11, 2019, and found that FDA did not consistently follow key procedures or meet key goals for its warning letter process. For example, when FDA issues a warning letter based on significant inspection violations, the agency has a goal to conduct a follow-up inspection within 6 months of the date the warning letter was issued. Of the 167 warning letters we reviewed, 125 were based on significant inspection violations. FDA met its 6-month goal for 14 (11 percent) of these 125 letters. For 56 (45 percent) of these letters, FDA conducted a follow-up inspection more than 6 months after the warning letter was issued—on average, about 2 years. For the remaining 44 percent, FDA had not conducted a follow-up inspection, as of March 11, 2020.







